One of the most common tips for using certain spices is to cook them in fat. Why does this matter? Flavour!
It is easy to think that flavour happens only in the tongue. Part of it does concern the classical tastes sensed by taste buds in the tongue and mouth - sweet, salty, sour, bitter, and umami. But flavour goes beyond that. One of its main components is aroma, which is sensed through olfactory receptors. In short, olfactory receptors in the nose and back of the mouth detect volatile (easily evaporated) molecules that are released from the food as we eat.
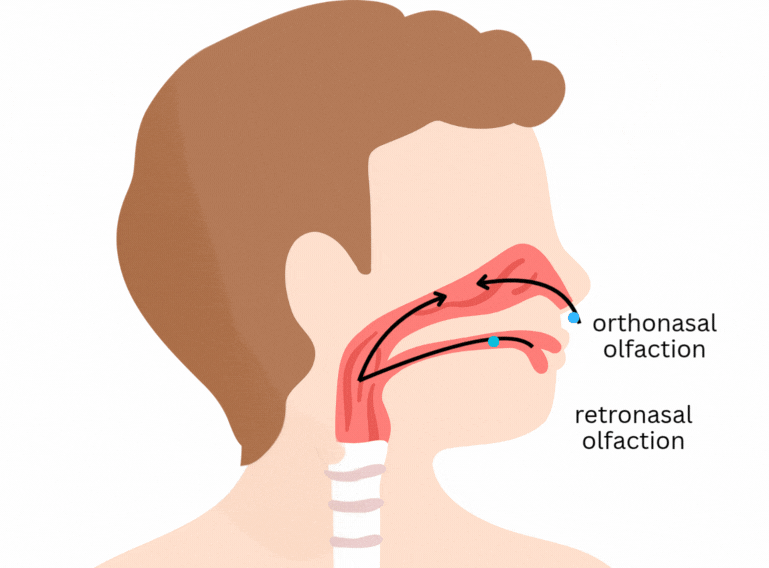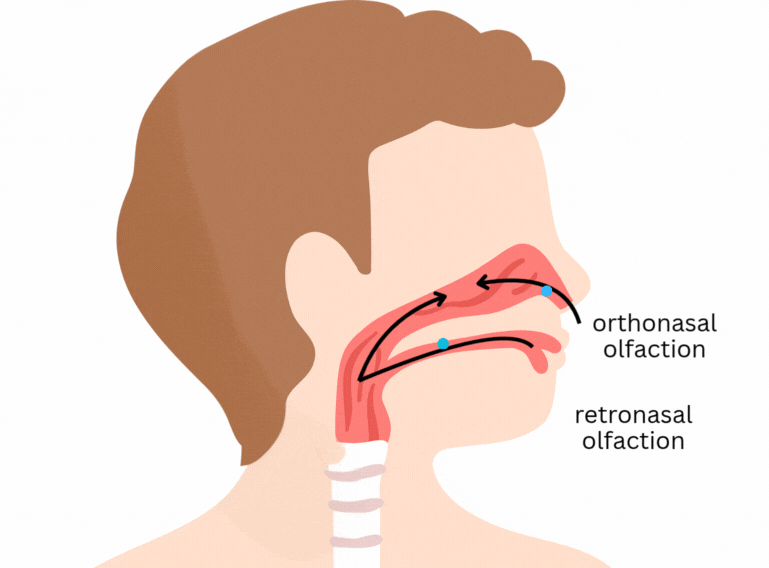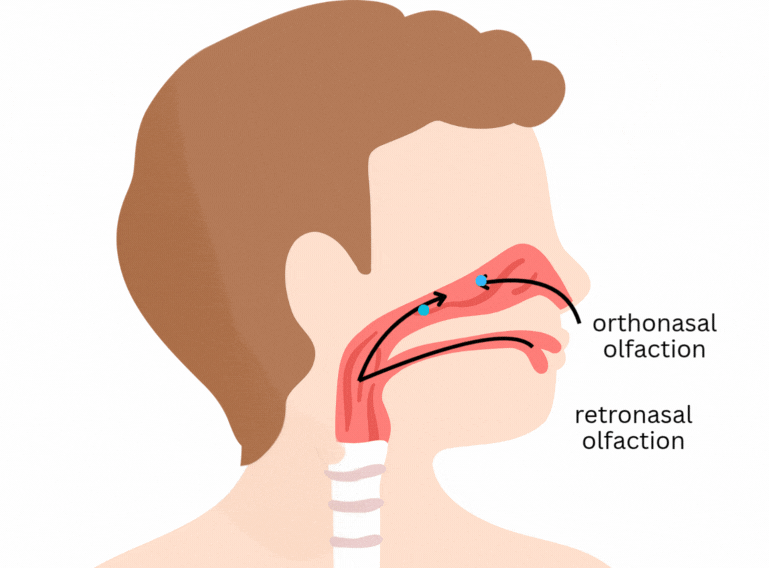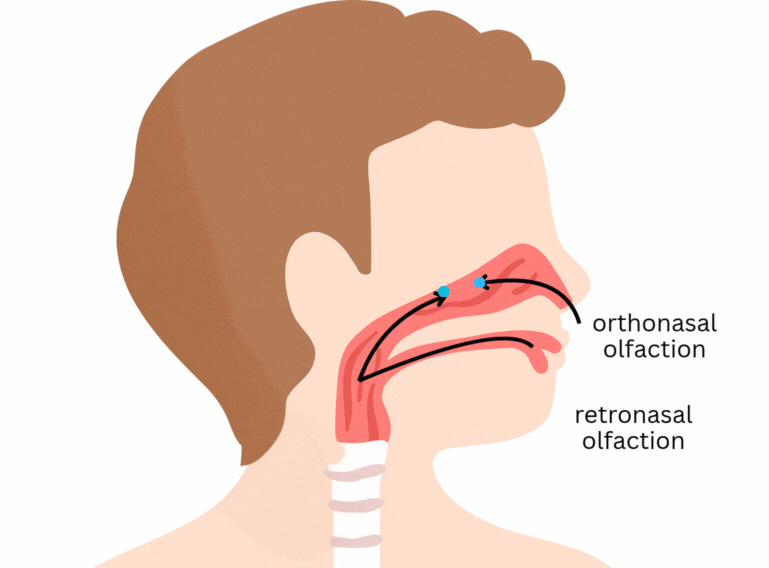
Here’s a previous post explaining some more about flavour:
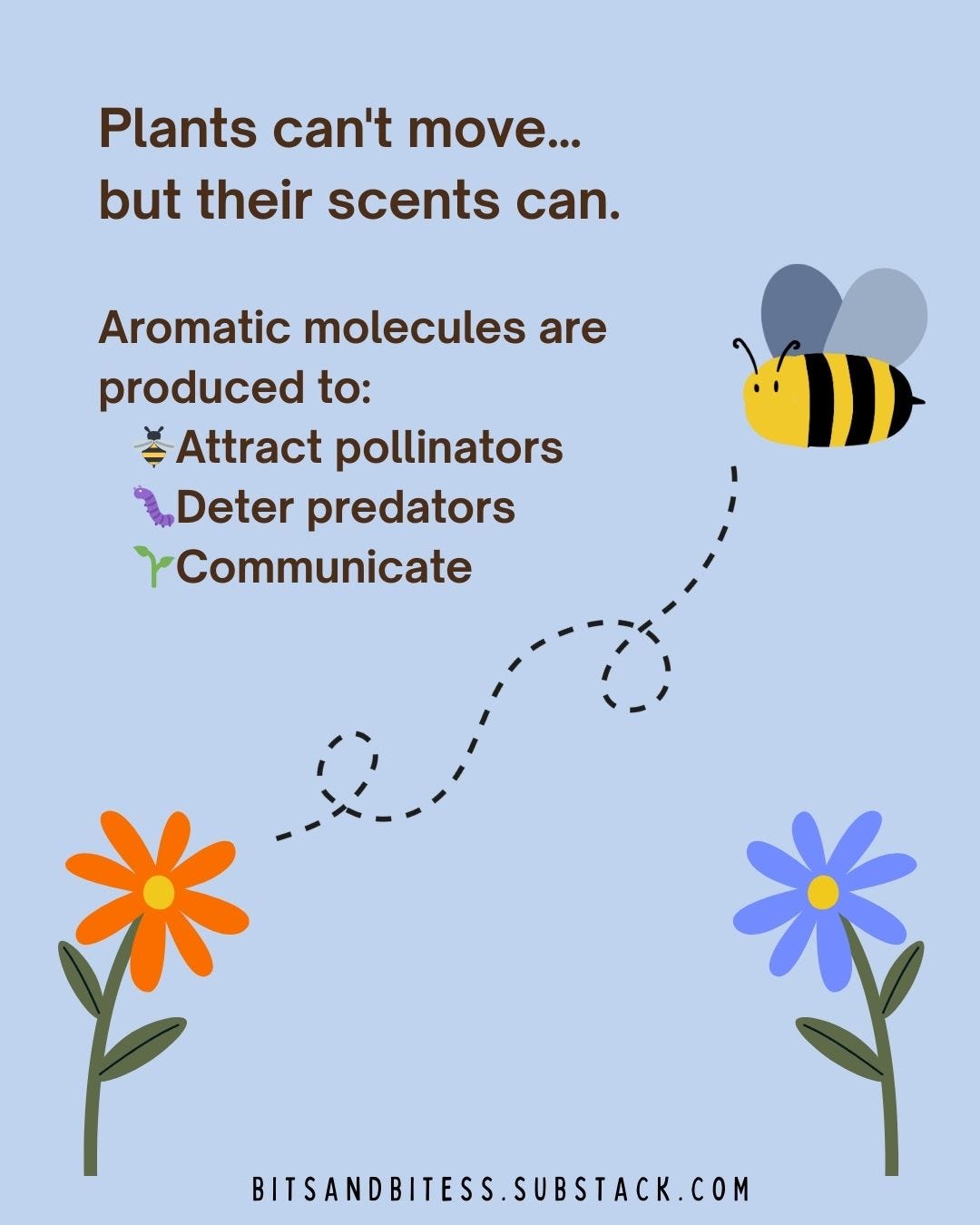
Spices and herbs are especially packed with aromatic volatile molecules. This is advantageous for them because, even though plants cannot move much, their aromas can span longer distances through the air and thus perform their intended functions: attract pollinators, keep predators away, and communicate with other plants12. To humans, many of these plant compounds end up being beneficial. For example, a compound that protects plants from microbes can work as a natural antibiotic for humans3.
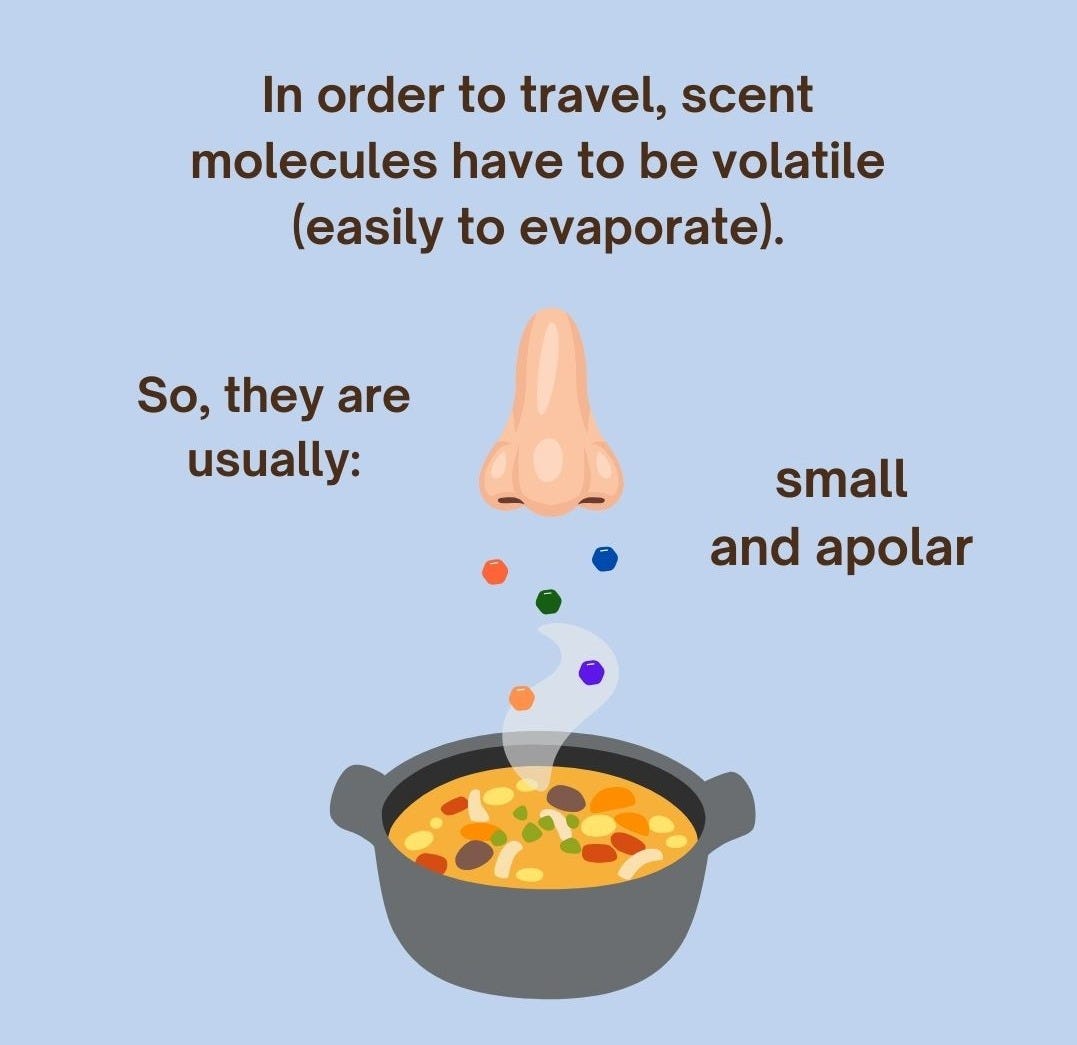
Molecules that evaporate easily are usually small and apolar4, the latter meaning that they don’t have much charge because electrons are distributed evenly throughout them5. Consequently, they don’t form strong bonds between themselves or with other molecules and, therefore, are freer to roam (in this case, evaporate). Apolar molecules are also less soluble in water (which is polar) and more soluble in fat (which has at least an apolar part).
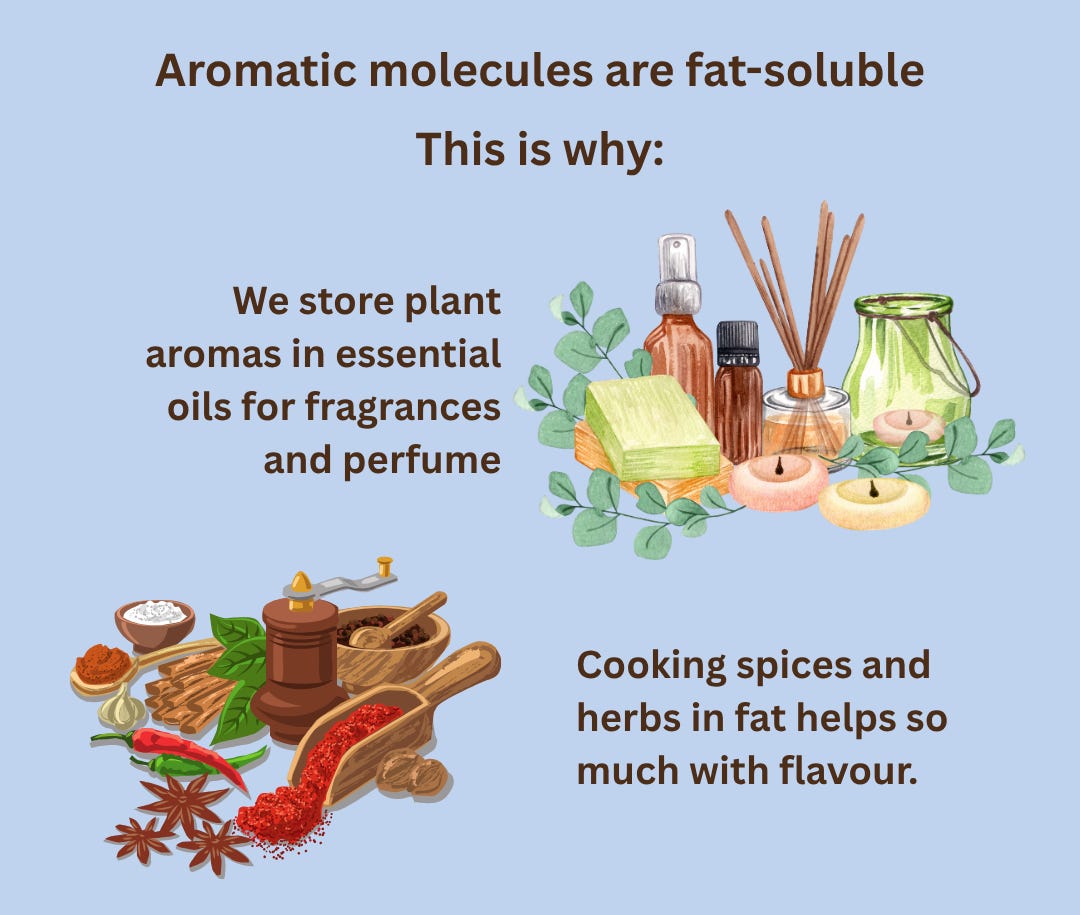
So, the aromatic compounds of plants, which are made to evaporate easily and not get dispersed in the water flowing through the plant, are mostly small, apolar, and fat-soluble molecules. You see why we often store plants’ pleasant aromas in essential oils for perfumes or fragrances?
But let’s go back to flavour! Since spices and herbs are packed with aromatic compounds, they can add a great deal of flavour to food. However, to make the most of their flavour, the pleasant aromatic molecules must be extracted and dispersed in the food. The best strategy is to use a medium in which they can dissolve.
Depending on its molecular structure, each compound will more easily disperse in a specific solvent. When cooking, solvents can be fat, water, or alcohol. Apolar molecules dissolve better in fat; polar molecules dissolve better in water; and amphiphilic molecules (big molecules with polar and apolar parts) dissolve better in alcohol6.
Since spices and herbs have mostly apolar aromatic compounds, it helps to cook them in a bit of fat. This way, their flavourful compounds will dissolve and distribute in the cooking medium. The flavourful fat will then coat the other ingredients and spread the flavour throughout the preparation. This process also happens when making chilli oil, garlic oil, or basil oil, and the oil retains the flavour even after those ingredients are strained out.
In essence, fat takes the flavour molecules where they need to go.
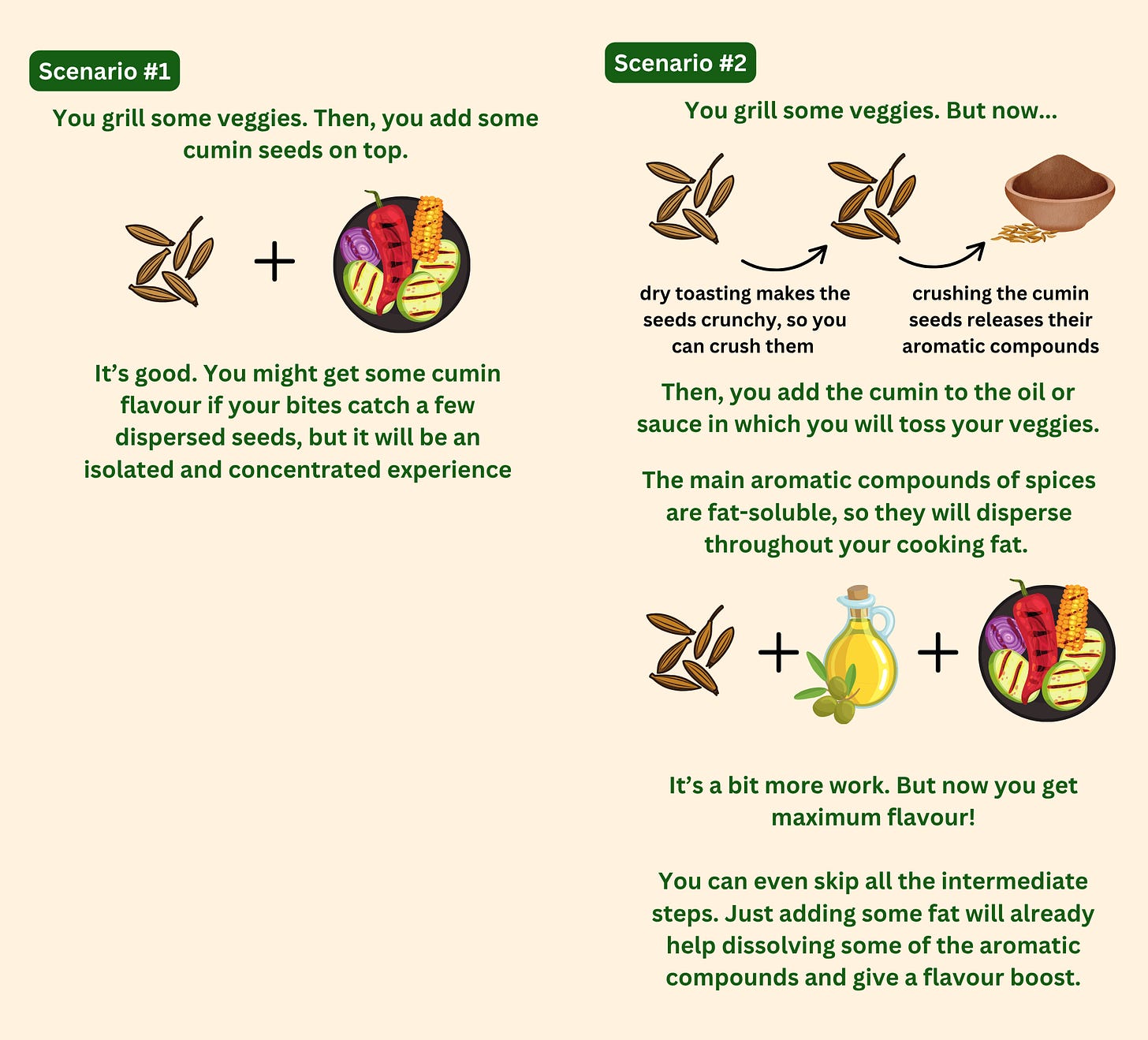
Let’s look at an example. If you directly add some cumin seeds to grilled veggies, you can get some cumin flavour when your bite happens to catch one of the dispersed seeds, but this will be an isolated and concentrated experience. If, instead, you dry toast some cumin seeds, crush them, and add them to the oil or sauce that will coat the veggies, the flavour will be dispersed in the fat and coat the surface of the veggies. When you take that bite, the flavour disperses in your mouth. This time, it is not just quickly passing through - the flavour is almost part of the veggies, and it lingers longer. The combined aromas will slowly but surely travel through the back of the mouth to the olfactory receptors and voilá - flavour floods your senses.
Stay tuned for the second part of this post, where we will learn other ways that fat can influence flavour, along with some tips for cooking with fat!
If there is anything you would like me to share or learn about, let me know in the comments! As always, you can also support my work by sharing it, subscribing, or even buying me a coffee.
But most of all, thank you for staying around and reading!
https://www.openaccessjournals.com/articles/aromatic-plants-natures-fragrant-bounty-and-their-diverse-applications-17054.html
https://kids.frontiersin.org/articles/10.3389/frym.2022.658692
https://link.springer.com/article/10.1007/s11101-018-9591-z
https://www.open.edu/openlearncreate/mod/oucontent/view.php?id=56488§ion=7
https://chem.libretexts.org/Courses/University_of_Arkansas_Little_Rock/Chem_1402%3A_General_Chemistry_1_%28Belford%29/Text/8%3A_Bonding_and_Molecular_Structure/8.7%3A_Bond_Polarity_and_Electronegativity_%28%28chi%29%29
https://www.khanacademy.org/science/hs-chemistry/x2613d8165d88df5e:solutions-acids-and-bases/x2613d8165d88df5e:solubility/v/solubility-and-intermolecular-forces





😋😋😋😋